Product Informations
Berberine hydrochloride, also known as berberine hydrochloride, has long been used in clinic as a heat clearing, detoxifying and antibacterial drug. It is commonly used to treat bacillary dysentery, acute gastroenteritis, chronic cholecystitis, conjunctivitis and suppurative otitis media. Berberine hydrochloride is an isoquinoline alkaloid, which exists in many plants of 4 families and 10 genera, such as Berberidaceae. Berberine can precipitate yellow acicular crystals from ether. Berberine is a quaternary ammonium alkaloid, and its salts have low solubility in water. For example, hydrochloride is 1 ∶ 500 and sulfate is 1 ∶ 30.
1、 Effect of berberine hydrochloride
Berberine hydrochloride has inhibitory effects on Shigella, Escherichia coli, Diplococcus pneumoniae, Staphylococcus aureus, Streptococcus, typhoid bacillus and Amoeba protozoa. Clinically, it is mainly used for intestinal infection and bacillary dysentery. It is also found that this product has the effect of anti arrhythmia. Berberine has strong antitumor activity in vivo and in vitro and can induce the differentiation of B16 cells; It has synergistic effect with cytarabine hydrochloride in vitro.
Berberine has antibacterial effect on hemolytic streptococcus, Staphylococcus aureus, Neisseria gonorrhoeae, Shigella flexneri and Shigella dysentery, and can enhance the phagocytosis of white blood cells. Berberine hydrochloride (commonly known as berberine hydrochloride) has been widely used in the treatment of gastroenteritis and bacterial dysentery. It also has a certain effect on pulmonary tuberculosis, scarlet fever, acute tonsillitis and respiratory tract infection.
It has a wide antibacterial spectrum and has antibacterial effect on a variety of gram-positive and negative bacteria in vitro, including hemolytic streptococcus, Staphylococcus aureus and Vibrio cholerae. Meningococci, Shigella dysentery, typhoid bacillus and Diphtheria Bacillus have strong inhibitory effects. They inhibit bacteria at low concentration and sterilize at high concentration. It can also inhibit influenza virus, amoeba protozoa, leptospira and some skin fungi. In vitro experiments confirmed that berberine can enhance the phagocytosis of leukocytes and hepatic reticuloendothelial system. Dysentery bacilli, hemolytic streptococcus, Staphylococcus aureus, etc. are very easy to develop drug resistance to this product. This product has no cross resistance with penicillin, streptomycin, etc.
2、 Application fields of berberine hydrochloride
Pharmaceutical industry: antibiotics are used to treat intestinal infection caused by dysentery bacilli.
Product Parameters
| COMPANY PROFILE | |
| Product Name | Berberine hydrochloride |
| CAS | 633-65-8 |
| Chemical Formula | C20H18ClNO4 |
| Brand | Hande |
| Manufacturer | Yunnan Hande Bio-Tech Co., Ltd. |
| Country | Kunming, China |
| Established | 1993 |
| BASIC INFORMATION | |
| Synonyms | TIMTEC-BB SBB006488;NATURAL YELLOW 18 CHLORIDE;NATURAL YELLOW 18;UMBELLATINE;BERBERINE HCL;BERBERINE HYDROCHLORIDE;BERBERIN HCL;BERBERINE HYDROCHLORIDE N-HYDRATE |
| Structure | 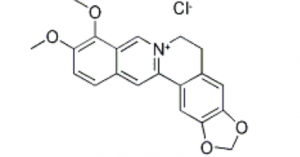 |
| Weight | 371.81 |
| HS Code | N/A |
| Quality Specification | Company Specification |
| Certificates | N/A |
| Assay | Customized according to customer needs |
| Appearance | Yellow block |
| Method of Extraction | Coptis chinensis |
| Annual Capability | Customized according to customer needs |
| Package | Customized according to customer needs |
| Test Method | HPLC |
| Logistics | Multiple transports |
| Payment Terms | T/T, D/P, D/A |
| Other | Accept customer audit all the time; Assist clients with regulatory registration. |
Hande product statement
1.All products sold by the company are semi-finished raw materials. The products are mainly aimed at manufacturers with production qualifications, and raw materials are not final products.
2.The potential efficacy and applications involved in the introduction are all from the published literature. Individuals do not recommend direct use, and individual purchases are refused.
3.The pictures and product information on this website are for reference only, and the actual product shall prevail.






