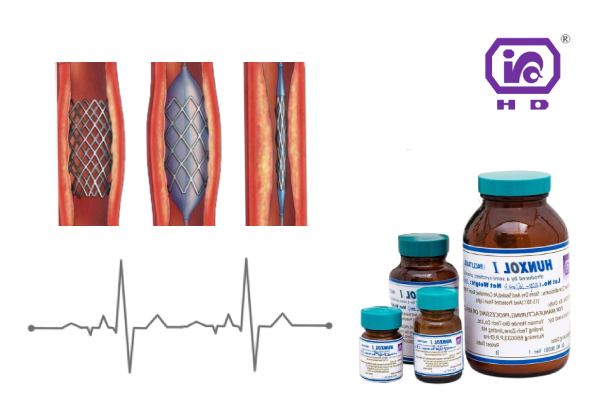
Different from the application of anti-tumor preparations, medical devices using paclitaxel are often high-risk interventional devices, including current drug-eluting stents, drug balloons, etc. Controlling quality risks is a matter of life and death. In particular, it is necessary for drugs to have higher quality standards, safer and better drug stability performance, and manufacturers to provide timely technical support under various scenarios in various stages of research and development, registration application, and mass production to match a variety of product development needs. The natural paclitaxel customized by Hande Bio for the combination of medicine and equipment can help enterprises solve related problems in an all-round way.
1. Advantages of Hande Bio-Natural Paclitaxel
1. Strict product quality control higher than China, EU and US Pharmacopoeia standards – high quality and strict release guarantee
2. Overseas registration and certification cover more than a dozen mainstream countries and regional markets – support the global development of customers
3. Customized testing items – matching the different technical requirements of customers
4. Customized packaging (minimum to 500mg) – convenient for customers to use in various scenarios
5. Technical support for the use of drugs – avoid drug waste caused by use under unsuitable conditions
6. Various research reports related to drugs – make the preparation of registration declaration more worry-free and time-saving
7. Global safe sales for 23 years, nearly 500 batches of products have been returned without quality problems – a stable, long-term reliable partner
2. Hande Bio provides CDMO one-stop service for the drugs used in the drug-device combination
1. Quickly customize R&D and provide samples according to customer needs
2. Establish methods, verification, quality research, testing, stability, cooperation declaration…
3. Use the fastest time and the most comprehensive service to support the customer’s product development strategy.
Extended reading: Yunnan Hande Biotechnology Co., Ltd. has been focusing on the production of paclitaxel for 26 years. It is the world’s first plant-derived anticancer drug paclitaxel approved by the US FDA, European EDQM, Australia TGA, China CFDA, India, Japan and other national regulatory agencies. Pharmaceutical independent manufacturer. Yunnan Hande natural Paclitaxel, available from stock, factory direct sales, welcome to inquire, 18187887160(WhatsApp same number).
Post time: Sep-23-2022