1. The latest work progress:
Regulatory declaration of natural paclitaxel:
● China CDE:
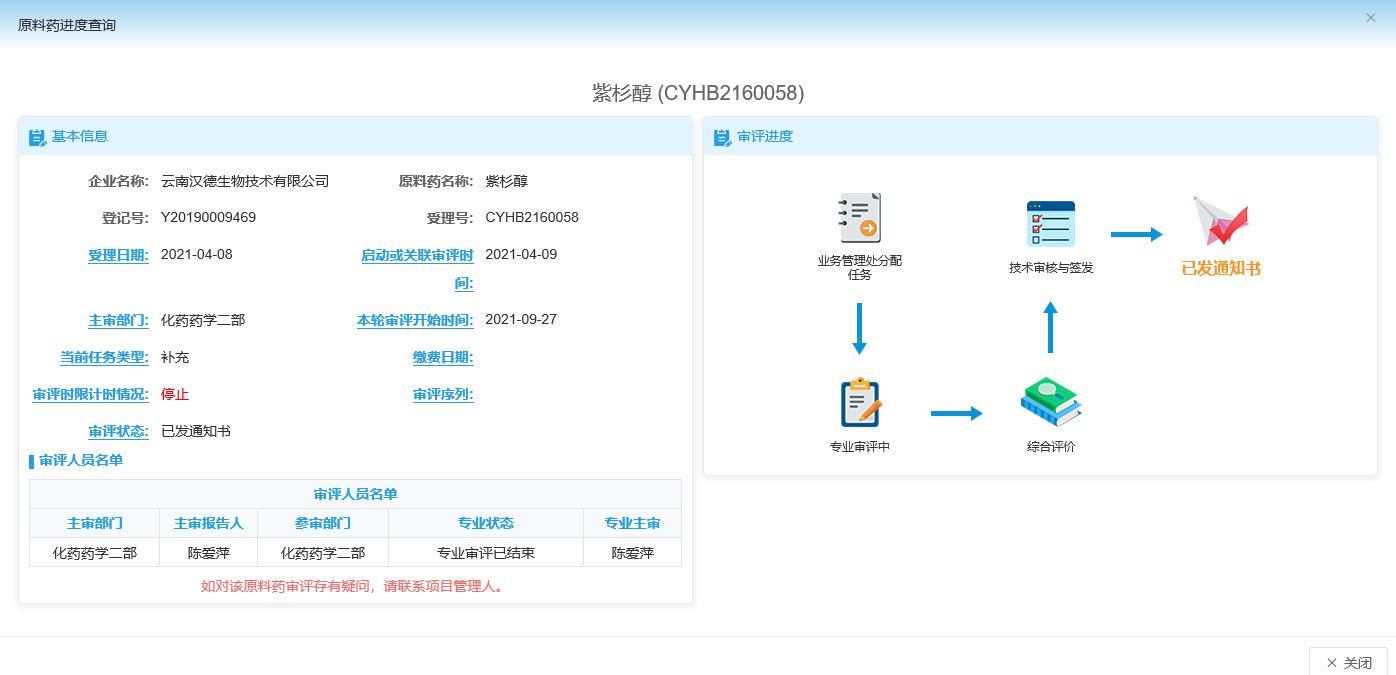
On December 13, 2021, the CDE supplementary application has been completed (the latest CDE status is shown in the pic). and Hande accepted the GMP on-site compliance inspection of Yunnan Provincial Food and Drug Administration on January 18-20, 2022. Since CDE will no longer issue any approval documents for API registration and change review, it will not issue any supporting documents for the time being, but it can already be referenced by preparations and devices. According to CDE’s approval, please refer to the change notification email for related process and standard changes. .
After the on-site inspection, Yunnan Hande will officially resume commercial sales in the Chinese market. Your company can use Hande’s products for research and development, small test, pilot test and clinical use according to your needs. Application materials can directly quote Hande CDE materials.
● EU EDQM:
Hande has obtained the CEP certificate and the “Certificate Document for Export of APIs to the EU” (“WC”), and the EU market has resumed official commercial sales. If your company needs CEP certificate and authorization letter or products, please contact us in time.
● US FDA:
The DMF change information has been submitted.
● Other countries or regions:
Russia: Existing customers have started to activate registration;
India: DMF data submitted;
Brazil: DMF data preparation;
Japan: Registration materials have been submitted;
Australian TGA: In preparation;
EU GMP: in preparation;
The rest of the countries will apply simultaneously. In the future, Hande will assist your company to expand the international strategic market.
2. 10-DAB semi-synthetic paclitaxel production line:
Pilot production is over and testing is completed. Now carry out quality research and stability inspection. The 3 batch verification batch production plan will start in January 2022 after the installation and commissioning of the equipment is completed.
10-DAB semi-synthetic paclitaxel regulatory registration plan:
● China CDE: Document No.: Y20210000685, it is planned to start the application in the second quarter of 2022;
● EDQM and FDA: Plan to file in the fourth quarter of 2022.
3. Primary processing line:
Through on-the-spot statistics and sampling testing of multiple yew planting bases, the annual procurement plan has been implemented with 3 large-scale planting bases to ensure sufficient raw material supply of effective amount of paclitaxel. The annual output of 10-DAB is 2000kg/year, with stable quality and long-term supply.
● 10-DAB III regulatory filing:
DMF: The DMF number has been obtained.
2. Other information
1. Affected by the epidemic, Hande will no longer participate in the CPHI exhibition. In order to better serve your company, we will continue to carry out customer visits and cooperate with your company’s market development work with better services.
Post time: Feb-18-2022